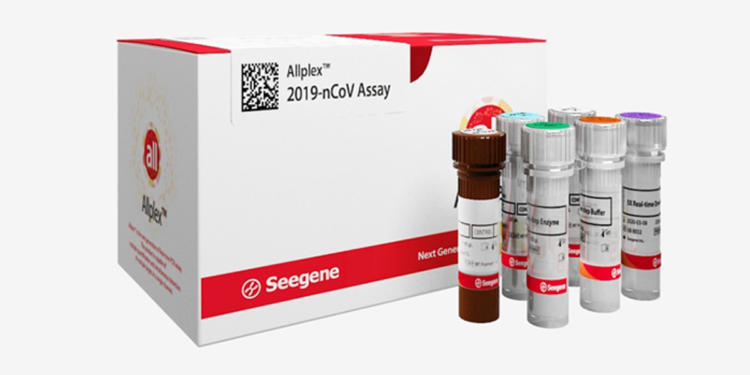
Seegene Inc., on Wednesday, announced that its COVID-19 test kit, Allplex™ 2019-nCoV Assay, received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA).
The molecular diagnostics company’s Real-time RT-PCR test for SARS-CoV-2 already sold over 10 million tests worldwide across 60 countries. In a single reaction tube, the RT-PDC assay could identify three different target genes, the E, RdRP, and N genes. This feature could maximize high-volume output with highly accurate results for COVISD-19 testing.
Seegene, with its exclusive AI-based assay design platform, rapidly developed the assay in the early weeks after the COVID-19 outbreak, which originated from China. The company already exports three million kits weekly, with plans to increase the weekly volume to five million starting May.
CEO Dr. Jong-Yoon Chun said that Seegene’s automated system, combined with its patented high multiplex chemistry technology, played a pivotal role in South Korea’s quick response to the pandemic. Thanks to its scalability and convenience in such situations, the company’s advanced analysis software proved extremely useful.
With the EUA approval from the FDA, Seegene anticipates major screening laboratories in the U.S. to start high-volume testing through its automated testing system.
Continuing Global Advocacy
The company also provides support for countries with inadequate infrastructure for RT-PCR testing. The Seegene Medical Foundation, South Korea’s largest diagnostics laboratory, can process up to 15,000 tests daily from samples received overseas.
Dr, Chun said that it is their duty to aid the global community in this pandemic. He added that they are proud to be able to provide Seegene’s diagnostic kits to multiple countries globally.
The company plans to expand its product to include enhanced performance that could detect even multiple mutations of the SARS-CoV-2. Dr. Chun said that they would continue to stay ready for any modifications needed to guarantee the most accurate detection.
Seegene’s reputation for its fast delivery of COVID-19 diagnostic kits attracted significant global demand. The company installed dedicated detection equipment in different countries based on various reagent products and an extensive global network.
Osang Healthcare early this week also received the U.S. FDA’s emergency use authorization for its RT-qPCR test kit named GeneFinder COVID-19 Plus RealAmp Kit.